
- Home
- Brand
- Memory
- Model
- Bcd325p2 (52)
- Bcd396xt (28)
- Bcd436hp (291)
- Bearcat (19)
- Cms600p2 (500)
- Cms600p2 Vet (23)
- Cms600p2-vet (79)
- Cms600p2vet (88)
- Ds3608-sr00003vzww (19)
- Handscan V8 (26)
- High Resolution (121)
- Pro-106 (39)
- Pro-651 (32)
- Pro-668 (41)
- Pro-96 (32)
- S50a (24)
- Sds100 (155)
- Trx-1 (128)
- V7 For Veterinary (21)
- Ws1040 (80)
- ... (2284)
- Series
- 1470g (2)
- Ds3508 (7)
- Ds3508-er (3)
- Ds3578 (2)
- Ds3608 (8)
- Ds3608-dp (2)
- Ds3608-sr (14)
- Ds4308 (5)
- Ds4308-hd (3)
- Honeywell Xenon 1950 (2)
- Li3608-er (3)
- Memor X3 (6)
- Motorola Ds3508 (3)
- Philips Dpm (3)
- Symbol Ds6707 (7)
- Symbol Ls2208 (2)
- Voyager 1472g (4)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (11)
- Zebra Li3608 (6)
- ... (3984)
- Type
- Bar Code Scanner (6)
- Barcode Scanner (118)
- Barcode Scanners (12)
- Calling System (30)
- Earpiece (9)
- Hand Held (22)
- Handheld (169)
- Handheld Scanner (44)
- Mobile (10)
- Mobile / In-vehicle (6)
- Mobile Scanner (8)
- Parts & Accessories (9)
- Portable (19)
- Portable / Handheld (662)
- Radio Scanner (8)
- Radio Scanners (9)
- Scanner (64)
- Ultrasound Machine (6)
- Ultrasound Scanner (10)
- White And Black (6)
- ... (2855)
- Wireless Range
Handheld Portable Full Digital Ultrasound Scanner Machine Convex Probe +oximeter



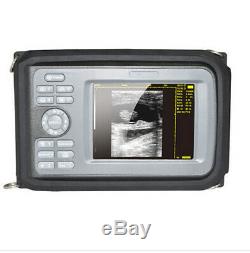



Display Modes: B, B+B, B+M, M, 4B. Image Conversion: distance, circumference, area, volume, EF rate, heart. Measurements: BPD, GS, CRL, FL, HC, AC, EDD, GA, FWVIDEO (PLAD, NTSC), USB2.0. One(1)R40/3.5mhz Convex Probe. Comments: date&time, name, sex, age, hospital.
Applications:Professional sofeware package in Obstetrics, Gynecology, Urology and Small parts. 100 (USD, EUR, AUD, GBP) so that we can make sure you get the. The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664; and CE Approved, TUV of Europe Cert. G1 10 02 50972 013. It takes approximately 5-7 days(USA , CA, AU, UK).
Other country 7-12 days European, South America Africa, etc. For a period of 24 months (24months for spare parts). The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) NumberK132989 The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) NumberK180353 The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) NumberK141973 massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) NumberK161892. 136606 510(k) Number K161892.The item "Handheld Portable Full Digital Ultrasound Scanner Machine Convex Probe +oximeter" is in sale since Tuesday, September 17, 2019. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "eshopoutlet88" and is located in Cranbury, New Jersey.
This item can be shipped to North, South, or Latin America, all countries in Europe, all countries in continental Asia, Australia.
- Brand: Does not apply
- Model: HIGH RESOLUTION
- MPN: Does not apply
- Country/Region of Manufacture: China
- UPC: 190891424792
- EAN: Does not apply
- Display: 5.5 inch TFT color LCD
- Express shipping: 3-5 Days
- Certificate: CE
- Detectable depth: 70mm~220mm
- Warrenty: 3 years
- With: R40/3.5mhz Convex Probe and L40/7.5Mhz HF linear Probe
- 1 box included: Yes

