
- Home
- Grayscale Depth
- Media Type
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 16 Gb (2)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4180)
- Scanner Type
- Technology
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (71)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3237)
12.1 Handheld Digital ultrasound Ultrasound Scanner 3.5Mhz Convex Probe FDA CE

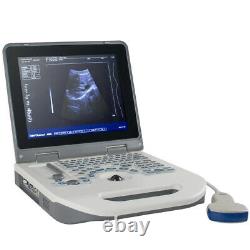

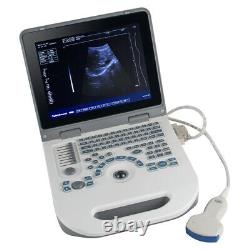








12.1 Handheld Digital ultrasound Ultrasound Scanner 3.5Mhz Convex Probe FDA CE. Probe configuration : T35R60BN convex array probe (standard); T65R13BN type cavity probe (user selected). 3T35R60BN convex array probe: B, B/B, 4B, B+M, M.
T65R13BN type cavity probe: B. T35R60BN convex array probe, Type B, real-time state:×0.8, ×1.0, ×1.2, × 1.5×1. ×0.8, ×2.0 (display penetration depth).
T65R13BN type cavity probe: ×0.8, ×1.0, ×1.2, ×1.5. M type speed : M type scanning has eight kinds of speed can be selected. Chinese and English translation : Transformation in both English and Chinese in interface. Zoom : Local x 2.0 magnification rate. Frequency :T35R60BN convex array probe real-time scan:2.5MHz/3.0MHz/3.5MHz/4.0MHz/5.0MHz five periods of frequency conversion.
T65R13BN type cavity probe real-time scan: 5.5 MHz/6.0 MHz/6.5 MHz/7.0 MHz/7.5MHz five periods of frequency conversion. Focus position : Send: 1, 2, 3 and 4-segments dynamic electron focusing; when 12-segments focus.The focus can be moved. Receive: receive real-time dynamic focus by focus. Measuring function : Distance, circumference/area (method of ellipse, method of loci), volume, heart rate, gestational weeks (BPD, GS, CRL, FL, HCAC), expected date of confinement and fetus weight, etc. Annotation function : hospital name, patient's name, age and gender. 64 body marks (with probe's position); Full-screen character annotation; Real-time clock display.
Puncture guide : 3.5MHz convex array probe can display puncture guide line in B mode. Gain control : 8 segments TGC and overall gain can be adjusted respectively. Image polarity :left and right flip, black and white flip, up and down flip. Image playback : series playback or check one by one.
Correction, frame correlation, point correlation, line correlation, digital filtering, digital edge enhancement and pseudo color processing, etc. Longitudinal geometric location accuracy:=8 %. Monitor: 12.1inch LED display. The perimeter area measurement deviation: 20% or less.
The battery pack continuous working time: =1h. Host weight: about 3.5 kg. Host appearance size: 310 ×338 × 76 (length × width × height) (mm3). FDA Declaration and CE certificate.
The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664; and CE Approved, TUV of Europe Cert. G1 10 02 50972 013.
You can consult with the FDA's Center for Devices and Radiological Health. All of our products are CE approved. Please kindly check the copy of the certificate in the listing below. It takes approximately 15-25 days(USA , CA, AU, UK). Other country 12-35 days European, South America, Africa, etc.
You pay us what you see on the invoice, i. We believe our items are so outstanding. All products are quality checked. We will be happy to resolve any issues you may have in a cordial and friendly manner.
We appreciate your Postive Feedback, and will do the same in return. IMPORTANT INFORMATION ON OUR POLICY AND FEEDBACK. Feedback if you are satisfied with our items.We try to be fair and accurate with all of our product listings and descriptions. If there is a problem, we can work together to resolve it.
We strive to always be extremely fair and always try to work with reasonable customers. We will always make every attempt possible to resolve issues if we made a mistake. Please do not assume a mistake is intentional.Let us know if our service could be better! So , if we don't respond you timely, Pls be patient, we will reply you as soon as possible.
The item "12.1 Handheld Digital ultrasound Ultrasound Scanner 3.5Mhz Convex Probe FDA CE" is in sale since Tuesday, July 21, 2020. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "luckymall88" and is located in ShenZhen.
This item can be shipped to United States, United Kingdom.
- Brand: Unbranded
- MPN: Does Not Apply
- Model: S50A
- UPC: Does not apply
- EAN: Does not apply
- Warranty: 2 years
- Dynamic range: 0~120dB adjustable
- Frequency: 2.5MHz/3.0MHz/3.5MHz/4.0MHz/5.0MHz five periods
- Display modes: 3T35R60BN convex array probe: B, B/B, 4B, B+M, M
- monitor: 12.1inch LED display
- Grey: 256 levels.
- Probe frequency: 3.5MHz
- Permanent Storage: 128 images.
- Zoom: Local x 2.0 magnification rate
- Scan Depth: 170mm
- Scanning Mode: electron scanning
- CE certified: FDA CE
- Appearance size:: 310 ×338 × 76 (length × width × height) (mm3)
- Host weight: about 3.5 kg.
- Image Colour: Black & White

