
- Home
- Brand
- Connectivity
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4178)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2851)
- Series
- Ds3508 (12)
- Ds3508-er (5)
- Ds3578 (6)
- Ds3608 (10)
- Ds3608-sr (21)
- Ds4308 (7)
- Ds4308-hd (3)
- Li3608-er (5)
- Memor X3 (6)
- Motorola Ds3508 (3)
- Philips Dpm (5)
- Symbol Ds6707 (8)
- Symbol Ls2208 (3)
- U1-d (4)
- Voyager 1472g (5)
- Voyager Xp 1472g (3)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (15)
- Zebra Ds9208 (5)
- Zebra Li3608 (18)
- ... (4675)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (70)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3234)
12 Portable Laptop 3D Digital Ultrasound Scanner Machine + 3.5MHZ Convex Probe
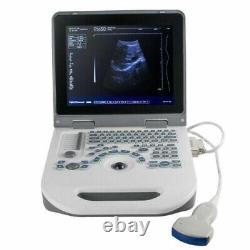
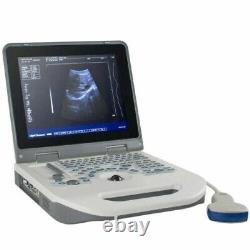
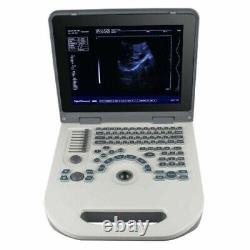




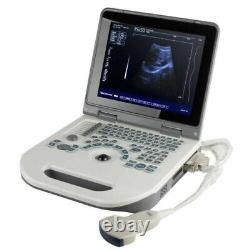


Probe configuration : T35R60BN convex array probe (standard); T65R13BN type cavity probe (user selected). 3T35R60BN convex array probe: B, B/B, 4B, B+M, M. T65R13BN type cavity probe: B. T35R60BN convex array probe, Type B, real-time state:×0.8, ×1.0, ×1.2, × 1.5, ×1. ×0.8, ×2.0 (display penetration depth). T65R13BN type cavity probe: ×0.8, ×1.0, ×1.2, ×1.5. M type speed : M type scanning has eight kinds of speed can be selected.
Chinese and English translation : Transformation in both English and Chinese in interface. Zoom : Local x 2.0 magnification rate. Frequency :T35R60BN convex array probe real-time scan:2.5MHz/3.0MHz/3.5MHz/4.0MHz/5.0MHz five periods of frequency conversion. T65R13BN type cavity probe real-time scan: 5.5 MHz/6.0 MHz/6.5 MHz/7.0 MHz/7.5MHz five periods of frequency conversion.
Focus position : Send: 1, 2, 3 and 4-segments dynamic electron focusing; when 1? The focus can be moved. Receive: receive real-time dynamic focus by focus. Measuring function : Distance, circumference/area (method of ellipse, method of loci), volume, heart rate, gestational weeks (BPD, GS, CRL, FL, HC?
AC), expected date of confinement and fetus weight, etc. Annotation function : hospital name, patient's name, age and gender. 64 body marks (with probe's position); Full-screen character annotation; Real-time clock display.Puncture guide : 3.5MHz convex array probe can display puncture guide line in B mode. Gain control : 8 segments TGC and overall gain can be adjusted respectively. Image polarity :left and right flip, black and white flip, up and down flip. Image playback : series playback or check one by one. Correction, frame correlation, point correlation, line correlation, digital filtering, digital edge enhancement and pseudo color processing, etc.
Monitor: 12.1inch LED display. The perimeter area measurement deviation: 20% or less. The battery pack continuous working time:? Host weight: about 3.5 kg.
Host appearance size: 310 ×338 × 76 (length × width × height) (mm3). The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is certified with the US FDA 510K No.
K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number. The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number.
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number. Massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number.

