
- Home
- Brand
- Item Height
- Item Length
- Item Width
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (335)
- Bearcat (25)
- Cms600p2 (512)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzww (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2880)
- Mounting
15 Veterinary Ultrasound Scanner Color Doppler Laptop Machine VET Rectal Probe
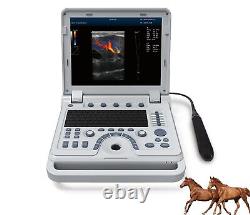
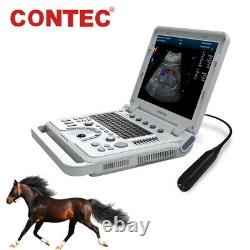



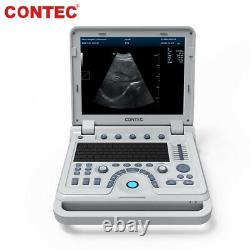
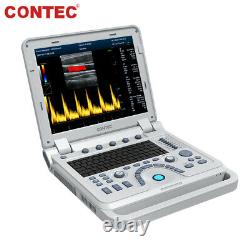
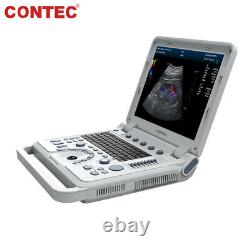



Color Doppler Veterinary Ultrasound Scanner Rectal Probe. CMS1700A-VET is a color Doppler ultrasonic diagnostic device with high resolution, which has a powerful computer processing platform.
It adopts Doppler ultrasound imaging technology, advanced image processing technology such as digital beam-forming technology, tissue harmonic imaging (THI), image speckle suppression, etc. And digital integrated graphic management system, and the internal professional measurement software package can fully meet the clinic diagnostic requirement. Laptop, slim design, smart and light, easy to carry, convenient for examining. 15 High resolution color LED monitor, high brightness, high contrast, wide visual, image clear and exquisite.Menu operation, Interface language: Chinese/English can be switched. OB measurement: EDD and GA for Bovine, equine, ovine, canine, feline, goat, swine and llama. Automatically calculate backfat and lean percentage of swine. Light touch keyboard, trackball and encoder, easy operation for doctor.
High speed USB port support high-capacity USB disk, and support the color laser printer print out all kinds of image and reports which make the output of diagnosis more convenient and simple. Linear array deflection/Trapezoidal imaging (optional).
Pulse inversion tissue harmonic imaging technology (iTHI). 1Display depth: = 300 mm.2Extended interface: VIDEO interface, S-VIDEO interface, RJ-45 interface, USB interface, VGA interface. Type of protection against electric shock: class? Degree of protection against electric shock: type B applied part.
Operating voltage: AC 100 V240 V. Operating frequency: 50 Hz/60 Hz. Power consumption: = 100 VA. Endo-rectal probe (7.5 MHz). Dimension: 370 mm (L) × 360 mm (W) × 80 mm (H). Included for REFERENCE: The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923. And certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. Note: For your earlier and safer to receive the item, please leave us your detailed address and telephone number. International Buyers - Please Note. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "contec_medical_systems" and is located in this country: US. This item can be shipped to United States.- Mounting: Laptop/Portable
- Brand: CONTEC
- Image Dimension: Two Dimensional
- Ultrasound Machine Features: Color Doppler
- Model: CMS1700A-VET
- Image Color: Color
- MPN: 69450401
- Country/Region of Manufacture: China
- Intended Use/Discipline: Obstetrics & Gynecology

