
- Home
- Brand
- Connectivity
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4178)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2851)
- Series
- Ds3508 (12)
- Ds3508-er (5)
- Ds3578 (6)
- Ds3608 (10)
- Ds3608-sr (21)
- Ds4308 (7)
- Ds4308-hd (3)
- Li3608-er (5)
- Memor X3 (6)
- Motorola Ds3508 (3)
- Philips Dpm (5)
- Symbol Ds6707 (8)
- Symbol Ls2208 (3)
- U1-d (4)
- Voyager 1472g (5)
- Voyager Xp 1472g (3)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (15)
- Zebra Ds9208 (5)
- Zebra Li3608 (18)
- ... (4675)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (70)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3234)
5.5 Handheld Ultrasound Scanner/Machine Digital +Convex Probe+Case for Human


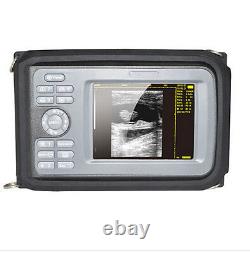





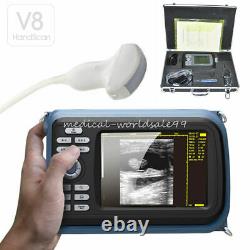

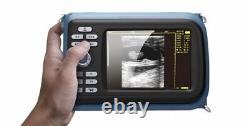


Handheld Ultrasound Scanner Convex Abdominal Probe 64 images USB disk Storage US. Display Modes: B, B+B, B+M, M, 4B. Image Conversion: distance, circumference, area, volume, EF rate, heart. Measurements: BPD, GS, CRL, FL, HC, AC, EDD, GA, FWVIDEO (PLAD, NTSC), USB2.0.
One(1)R40/3.5mhz Convex Probe. Comments: date&time, name, sex, age, hospital.
Applications:Professional sofeware package in Obstetrics, Gynecology, Urology and Small parts. Food and Drug Administration and state and local regulatory agencies. Or replace item for you. W e commit ourself to do 100% customer satisfactione. We'll do our best to help you. The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664; and CE Approved, TUV of Europe Cert.G1 10 02 50972 013. You can consult with the FDA's Center for Devices and Radiological Health. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical/Lab Equipment Attachments & Accessories\Medical Sensors & Ultrasound Probes". The seller is "medicalshop888" and is located in this country: CN.
This item can be shipped worldwide.- Country/Region of Manufacture: China
- Model: HIGH RESOLUTION
- Gray scale: 256 levels
- Feature: Handheld Ultrasound scanner
- Display: 5.5 inch TFT color LCD
- Express shipping: Yes
- Frequency Range: 2.5MHZ-7.5MHZ
- Cline-loop: 400frames
- Display Mode:: B,B+B,B+M,M,4B
- Certificate:: CE FDA Passed
- Detectable depth: 70mm~220mm
- Warrenty: 2 years
- With: Carry Case and Belt
- data storage: Built-in memory-64 images USB disk storage
- Image Reservation: up/down,left/right
- Brand: unbranded

