
- Home
- Band
- 700 Mhz (2)
- Air (6)
- Am, Fm (6)
- Band (3)
- Cb, Uhf, Vhf (2)
- Digital (2)
- Hf, Uhf, Vhf (2)
- Hf, Vhf, Uhf (18)
- Hf, Vhf, Uhf, Cb (10)
- True I / Q Scanner (3)
- Uhf (169)
- Uhf, Hf (3)
- Uhf, Vhf (22)
- Uhf, Vhf, Cb (2)
- Uhf, Vhf, Hf (195)
- Uhf, Vhf, Hf, Cb (35)
- Uniden (4)
- Vhf (18)
- Vhf, Uhf (6)
- Vhf, Uhf, Cb (9)
- ... (4305)
- Brand
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (2)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4177)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2849)
- Resolution
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (70)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3232)
5.5 Handheld Ultrasound Scanner/Machine Digital +Convex Probe+Case for Human


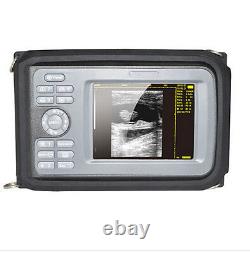





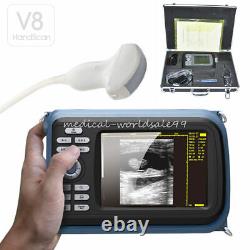

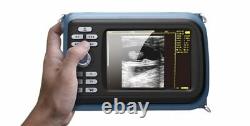


Handheld Ultrasound Scanner Convex Abdominal Probe 64 images USB disk Storage US. Display Modes: B, B+B, B+M, M, 4B. Image Conversion: distance, circumference, area, volume, EF rate, heart. Measurements: BPD, GS, CRL, FL, HC, AC, EDD, GA, FWVIDEO (PLAD, NTSC), USB2.0.
One(1)R40/3.5mhz Convex Probe. Comments: date&time, name, sex, age, hospital. Applications:Professional sofeware package in Obstetrics, Gynecology, Urology and Small parts. Food and Drug Administration and state and local regulatory agencies.
Or replace item for you. W e commit ourself to do 100% customer satisfactione. We'll do our best to help you.
The Fingertip Pulse-Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 136606, and certified by FDA of United States Premarket Submission Number (510K): K073454 Listing Number: D045684, K082641 Listing Number: D064765, K090671 Listing Number: D078664; and CE Approved, TUV of Europe Cert. G1 10 02 50972 013.
You can consult with the FDA's Center for Devices and Radiological Health. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical/Lab Equipment Attachments & Accessories\Medical Sensors & Ultrasound Probes".
The seller is "medicalshop888" and is located in this country: CN. This item can be shipped worldwide.
- Country/Region of Manufacture: China
- Model: HIGH RESOLUTION
- Gray scale: 256 levels
- Feature: Handheld Ultrasound scanner
- Display: 5.5 inch TFT color LCD
- Express shipping: Yes
- Frequency Range: 2.5MHZ-7.5MHZ
- Cline-loop: 400frames
- Display Mode:: B,B+B,B+M,M,4B
- Certificate:: CE FDA Passed
- Detectable depth: 70mm~220mm
- Warrenty: 2 years
- With: Carry Case and Belt
- data storage: Built-in memory-64 images USB disk storage
- Image Reservation: up/down,left/right
- Brand: unbranded

