
- Home
- Brand
- Connectivity
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4178)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2851)
- Series
- Ds3508 (12)
- Ds3508-er (5)
- Ds3578 (6)
- Ds3608 (10)
- Ds3608-sr (21)
- Ds4308 (7)
- Ds4308-hd (3)
- Li3608-er (5)
- Memor X3 (6)
- Motorola Ds3508 (3)
- Philips Dpm (5)
- Symbol Ds6707 (8)
- Symbol Ls2208 (3)
- U1-d (4)
- Voyager 1472g (5)
- Voyager Xp 1472g (3)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (15)
- Zebra Ds9208 (5)
- Zebra Li3608 (18)
- ... (4675)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (70)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3234)
CE CMS600B3 Full Digital Portable Ultrasound Scanner Machine, 3.5M Convex Probe
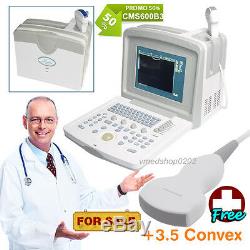



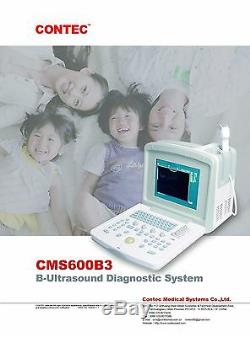




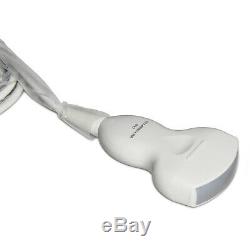


CE CMS600B3 Full Digital Portable Ultrasound Scanner Machine, 3.5M Convex Probe. This equipment is high resolution linear/convex ultrasound scanner. It adopts micro-computer control and digital scan converter (DSC), digital beam-forming (DBF), real time dynamic aperture (RDA), real time dynamic receiving apodization, real time dynamic receiving focusing (DRF), digital frequency scan (DFS), frame correlation technologies.
The device is suitable for ultrasonic examination on abdominal, obstetric, cardiac, small parts. PAL-D video output offers connection to external video image printer and big display and other equipments. High speed USB port provides real time image transfer to the PC. Adoption of folded soft push keyboard and trackball provides immediate, convenient and flexible operation. Field programmable gate array and surface mounted technology make this equipment compact and light in weight.
Jet molding enclosure and potable structure. Display mode: B, B/B, B/M, M, 4B, B+M/M. Image gray scale: 256 Scale.
Depth of penetration: 40 mm-240 mm. Geometric: Horizontal 15 % Vertical 10 %.Resolution: Lateral 2mm (Depth80) 3mm (80 Depth130). Image conversion: Up/down, left/right, black/white. Interface: USB2.0, VIDEO, COM.
Measurement: Distance, circumference, area, volume, heart rate, GA, FW, EDD. 3.5 MHz Convex Probe. Probe Frequency: 2.5-5.0 MHz Applications: Abdominal organs examination. 7.5 MHz HF Linear Probe Probe Frequency: 6.5-8.5 MHz Applications: Small part examination.
5.0 MHz Micro-Convex Probe Probe Frequency: 4.0-5.5 MHz Applications: Cardiology examination. 6.5 MHz Transvaginal Probe Probe Frequency: 5.5-7.5 MHz Applications: Obstetrics and gynecology examination. 6.5 MHz Endorectal Linear Probe Probe Frequency:5.0-7.5 MHz Applications: Animal examination. Dimension: 304 (L) × 222 (W) × 289 (H) mm. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. These charges are buyers' responsibility. The item "CE CMS600B3 Full Digital Portable Ultrasound Scanner Machine, 3.5M Convex Probe" is in sale since Saturday, April 27, 2019.This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical/Lab Equipment Attachments & Accessories\Medical Sensors & Ultrasound Probes". The seller is "vmedshop0202" and is located in Qinhuangdao,Hebei,. This item can be shipped to North, South, or Latin America, all countries in Europe, all countries in continental Asia, Australia.
- Model: CMS600B3
- Country/Region of Manufacture: China
- MPN: 69450401
- Brand: CONTEC
- Standard probe: 3.5mhz convex probe
- optional probes: linear probe,micro-convex probe,transvaginal probe
- UPC: Does not apply

