
- Home
- Brand
- Connectivity
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4178)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2851)
- Series
- Ds3508 (12)
- Ds3508-er (5)
- Ds3578 (6)
- Ds3608 (10)
- Ds3608-sr (21)
- Ds4308 (7)
- Ds4308-hd (3)
- Li3608-er (5)
- Memor X3 (6)
- Motorola Ds3508 (3)
- Philips Dpm (5)
- Symbol Ds6707 (8)
- Symbol Ls2208 (3)
- U1-d (4)
- Voyager 1472g (5)
- Voyager Xp 1472g (3)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (15)
- Zebra Ds9208 (5)
- Zebra Li3608 (18)
- ... (4675)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (70)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3234)
CONTEC Cart Mounted Ultrasound Scanner Machine Color Doppler Convex Linear Heart
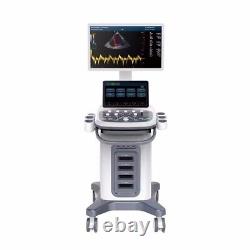
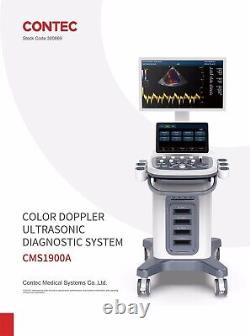

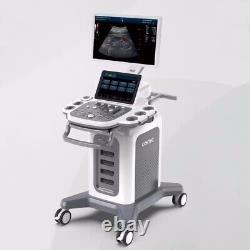

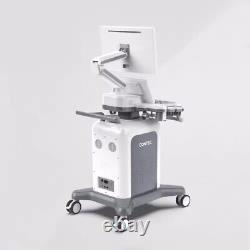

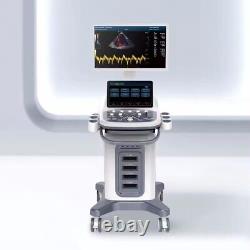


CONTEC CMS1900A Cart-mounted color Doppler ultrasound. CMS1900A is a color Doppler ultrasonic diagnostic device which has a computer processing platform. The system is mainly suitable for the diagnosis of abdomen, heart, peripheral vessels, breast, obstetrics and gynecology, small organs, urology, muscle, incretion and pediatrics, etc. It adopts Doppler ultrasound imaging technology, advanced image processing technology such as digital beam-forming technology, tissue harmonic imaging (THI), image speckle suppression, etc. And digital integrated graphic management system, and the internal professional measurement software package can fully meet the clinic diagnostic requirement.
23.8 inch display device; 13.3 inch touch screen. Equipped with couplantheating cup and standard USB keyboard.
Superior in phased rray cardiac imaging technology, complete in software package of cardiac measurement function. Sync implementation of three modes (B+CF/PD/DPDI+PW).
Extended Convex Array Imaging, Linear Array Deflection/Trapezoid Imaging. Color M-mode, Anatomic M mode.
Software packages: routine, abdomen, obstetrics, gynecology, vessels, small organ, urology, cardiac etc. Scan depth: 250 mm max. Extended interface: four probe interfaces, four USB interfaces. One VIDEO interface, one S-VIDEO interface, RJ-45 interface.Type of probe: Convex Probe, Micro Convex Probe, Trans-Rectal Probe, Trans Vaginal Probe, Linear Probe, Phased Array Probe, Volume Probe. Type of protection against electric shock: class I equipment.
Degree of protection against electric shock: type BF applied part. Operating voltage: AC 100V - 240V. Operating frequency: 50 Hz/60 Hz. One abdominal probe (3.5MHz).
One linear probe (7.5MHz). Food and Drug Administration and state and local regulatory agencies.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved.
