
- Home
- Grayscale Depth
- Media Type
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 16 Gb (2)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4180)
- Scanner Type
- Technology
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (71)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3237)
CONTEC Ultrasound Scanner Laptop PW Doppler Pseudo-color Function 7.5Mhz Linear
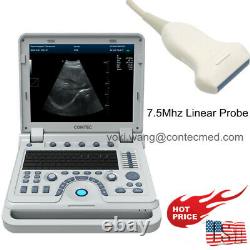
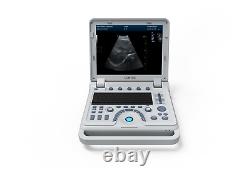


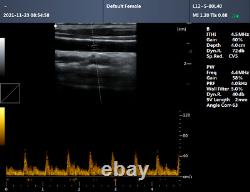
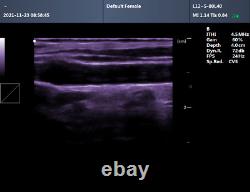
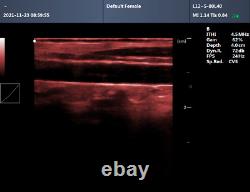
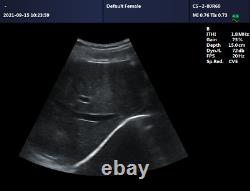


CMS600P2PLUS is a B-Ultrasound Diagnostic System with high resolution and PW Doppler. The system is mainly suitable for the ultrasonic examination of abdomen, obstetrics and gynecology, small organs, urology, etc. It adopts advanced image processing technology such as digital beam forming technology, tissue harmonic imaging (THI), image speckle suppression, etc. And digital integrated graphic management system, and the internal professional measurement software package can fully meet the clinic diagnostic requirement. Built-in lithium battery, the device can work continuously for up to 2 h after fully charged.
Store the patient data by 128 G hard disk. Advanced Digital Beam Forming (DBF) technology. Working mode: B, 2B, 4B, B/M, M, PW.
Software packages: routine, abdomen, obstetrics, gynecology, vessels, small organ, urology, etc. Scan depth: 250 mm max. Extended interface: two probe interfaces, two USB interfaces, one VIDEO interface, one S-VIDEO interface, RJ-45 interface, one VGA interface.
Type of probe: Convex Probe, Micro Convex Probe, Trans-Rectal Probe, Trans-Vaginal Probe, Linear Probe. U Type of protection against electric shock: class? U Degree of protection against electric shock: type BF applied part. U Operating voltage: AC 100 V 240 V. U Operating frequency: 50 Hz/60 Hz. U Power consumption: = 100 VA.370 mm (L) × 360 mm (W) × 80 mm (H). Weight: 6.5 k g. 1 examination of abdominal tissues including: liver, gallbladder, spleen, pancreas, kidney, uterus, bladder, prostate, etc. 2 assessment of fetal growth and fetal maturity in obstetric diagnosis. Be mainly used for examination of heart and abdominal tissues.
Be mainly used for examination of rectum. Be mainly used for gynecological examination. Be mainly used for neonate, musculoskeletal system, peripheral vessels, small organs breast, thyroid, testis, etc. The sale of this item may be subject to regulation by the U.
Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG).
With the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. Make sure you get the item safely. You can get the item in 10-25 days. You pay us what you see on the invoice, i.
That occurred for certain goods, or you may kindly give us some suggestions about that, we promise to do our best to help you! All return items must be.Or replace item for you free of charge. We guarantees new equipment other than accessories to be free from defects in workmanship and materials for a period of twelve months six months. Company's option, any part which upon our company's examination proves defective. Contec Medical Systems focusing on research, manufacture and distribution of medical instruments, was founded in 1992 as a high-tech company. At present there are more than 1200 employees in our company.
Our product line covers a wide range of 13. Most of the domestic hospitals are our customers. Contec hopes to cooperate with international. Companies to supply more innovative design and advanced technology products. We sincerely welcome you to become one of our global partners. We are looking forward to establishing a. Successful business relationship with you. We will try our best to help you to solve the problem and give you satisfied answer. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "michewan-0" and is located in this country: US. This item can be shipped to United States.- Model: CONTEC CMS600P2Plus
- Mounting: Laptop/Portable
- MPN: 69450401
- Image Color: Black & White
- Brand: CONTEC
- Ultrasound Machine Features: Color Doppler

