
- Home
- Brand
- Connectivity
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4178)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2851)
- Series
- Ds3508 (12)
- Ds3508-er (5)
- Ds3578 (6)
- Ds3608 (10)
- Ds3608-sr (21)
- Ds4308 (7)
- Ds4308-hd (3)
- Li3608-er (5)
- Memor X3 (6)
- Motorola Ds3508 (3)
- Philips Dpm (5)
- Symbol Ds6707 (8)
- Symbol Ls2208 (3)
- U1-d (4)
- Voyager 1472g (5)
- Voyager Xp 1472g (3)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (15)
- Zebra Ds9208 (5)
- Zebra Li3608 (18)
- ... (4675)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (70)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3234)
Digital Handscan Handheld Ultrasound Scanner Machine + Convex Probe + Battery US
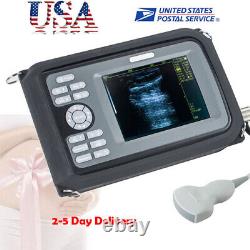
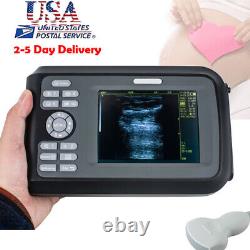

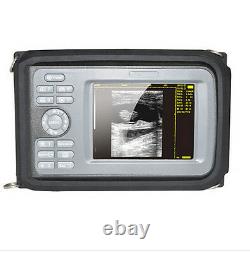










Display Modes: B, B+B, B+M, M, 4B. Image Conversion: distance, circumference, area, volume, EF rate, heart. Measurements: BPD, GS, CRL, FL, HC, AC, EDD, GA, FWVIDEO (PLAD, NTSC), USB2.0. One(1)R40/3.5mhz Convex Probe.
Comments: date&time, name, sex, age, hospital. Applications:Professional sofeware package in Obstetrics, Gynecology, Urology and Small parts. The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is certified with the US FDA 510K No.
K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number. K132989 The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number. K180353 The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number. K141973 massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number. All items are in brand new condition unless specified. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Other Healthcare, Lab & Dental".The seller is "greenhealthy2022" and is located in this country: US. This item can be shipped to United States.
- Model: HIGH RESOLUTION
- Country/Region of Manufacture: China
- Gray scale: 256 levels
- Feature: Handheld Ultrasound scanner
- Display: 5.5 inch TFT color LCD
- Express shipping: Yes
- Frequency Range: 2.5MHZ-7.5MHZ
- Cline-loop: 400frames
- Display Mode:: B,B+B,B+M,M,4B
- Certificate:: CE FDA Passed
- Detectable depth: 70mm~220mm
- Warrenty: 2 years
- With: Carry Case and Belt
- data storage: Built-in memory-64 images USB disk storage
- Image Reservation: up/down,left/right
- Probe:: R40/3.5mhz Convex Probe

