
- Home
- Band
- 700 Mhz (2)
- Air (6)
- Am, Fm (6)
- Band (3)
- Cb, Uhf, Vhf (2)
- Digital (2)
- Hf, Uhf, Vhf (2)
- Hf, Vhf, Uhf (18)
- Hf, Vhf, Uhf, Cb (10)
- True I / Q Scanner (3)
- Uhf (169)
- Uhf, Hf (3)
- Uhf, Vhf (22)
- Uhf, Vhf, Cb (2)
- Uhf, Vhf, Hf (197)
- Uhf, Vhf, Hf, Cb (35)
- Uniden (4)
- Vhf (18)
- Vhf, Uhf (6)
- Vhf, Uhf, Cb (9)
- ... (4313)
- Brand
- Isbn
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (25)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzww (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2858)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (71)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (841)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3239)
- Weight
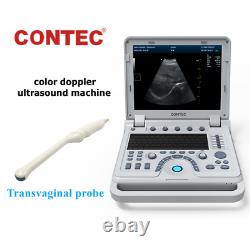
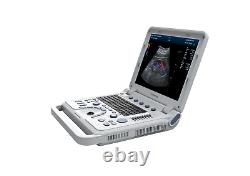
New Color Doppler Ultrasound Scanner Machine CF PW Transvaginal Probe CMS1700A





Integrated with SpO2 probe and processing display module 2. Light in weight and convenient in carrying 3. Operation of the product is simple, low power consumption 4.
Pulse rate value display, bar graph display 6. Low-voltage indicator appears before working abnormally which is due to low-voltage 7. Automatically power off function: it will automatically power off within 5 seconds if the finger falls out of probe.
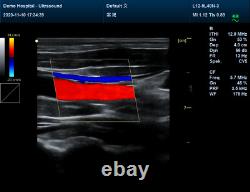
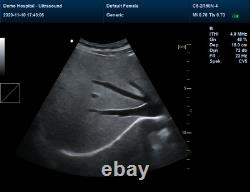
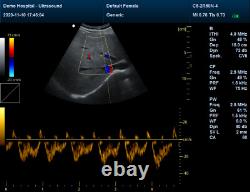
Display Mode:LED display 2. Power Supply:1.5V(AAA size)alkaline batteries×2 3. Battery working hour:Minimum continually work time is 24 hours, theoretical number is 56 hours Sell in standard 1.CMS1700A, Color Doppler Ultrasound Scanner Machine. CMS1700A is a color Doppler ultrasonic diagnostic device with high resolution, which has a powerful computer processing platform. The system is mainly suitable for the diagnosis of abdomen, heart, peripheral vessels, breast, obstetrics and gynecology, small organs, urology, muscle, incretion and pediatrics, etc.
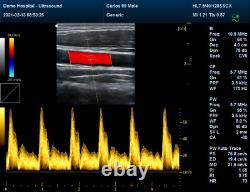
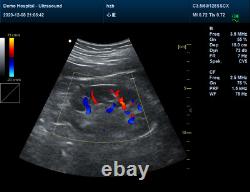
It adopts Doppler ultrasound imaging technology, advanced image processing technology such as digital beam-forming technology, tissue harmonic imaging (THI), image speckle suppression, etc. And digital integrated graphic management system, and the internal professional measurement software package can fully meet the clinic diagnostic requirement. Color Doppler (CF), color power Doppler imaging (PDI). B+CF Color double-frame real-time imaging. Linear array deflection/trapezoid imaging, contrast imaging. Display depth:= 300 mm. Sync implementation of three modes (B+CFM/PDI+PWD). Extended interface: VIDEOinterface, S-VIDEO interface, RJ-45 interface, USB interface, VGA interface. Type of protection against electric shock: class?Degree of protection against electric shock: type B applied part. Operating voltage: AC 100 V240 V. Dimension: 370 mm (L) × 360 mm (W) × 80 mm (H). Please pay within 2 days so as to avoid unnecessary delays and to ensure you get your item ASAP. An unpaid strike will be sent if the item has not been paid after 14 days.
Take up about 1 month due to strict custom inspection. May take up to 4-8 weeks due to strict custom inspection. 100% satisfaction is our goal! Client is reaponsible for the custom cleatance and custom duty.If you have a defective item, you want to return or discount. Food and Drug Administration and state and local regulatory agencies.
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. All emails will be answered within 24 hours on weekdays(but within 48hours on weekends). Only English user guide, if you need any other languange, please contact me. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "wonderfuldays365" and is located in this country: CN. This item can be shipped worldwide.- Model: CMS1700A
- Country/Region of Manufacture: China
- Mounting: Laptop/Portable
- MPN: 69450401
- Brand: CONTEC
- Intended Use/Discipline: Small Organs, Peripheral Vessels, Abdomen, Cardiology, Obstetrics & Gynecology, Pediatrics, Urology
- Ultrasound Machine Features: Convex Probe, Color Doppler

