
- Home
- Band
- 700 Mhz (2)
- Air (6)
- Am, Fm (6)
- Band (3)
- Cb, Uhf, Vhf (2)
- Digital (2)
- Hf, Uhf, Vhf (2)
- Hf, Vhf, Uhf (18)
- Hf, Vhf, Uhf, Cb (10)
- True I / Q Scanner (3)
- Uhf (169)
- Uhf, Hf (3)
- Uhf, Vhf (22)
- Uhf, Vhf, Cb (2)
- Uhf, Vhf, Hf (196)
- Uhf, Vhf, Hf, Cb (35)
- Uniden (4)
- Vhf (18)
- Vhf, Uhf (6)
- Vhf, Uhf, Cb (9)
- ... (4312)
- Brand
- Features
- Battery Operated (4)
- Channel (3)
- Channel Monitor (42)
- Copier, Portable (8)
- Ctcss (19)
- Dcs (5)
- Digital (7)
- Digital Display (8)
- Digital Handheld (4)
- Gps (9)
- Handy Receiver (3)
- Motorola (4)
- P25 (3)
- Portable (10)
- Portable, Scanner (15)
- Printer (3)
- Radio (14)
- Scanner (17)
- Voice Activated (4)
- Wi-fi (6)
- ... (4642)
- Memory
- 100 Channels (193)
- 1000 Channels (240)
- 128 Channels (21)
- 16 Gb (2)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4181)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (25)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2856)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (71)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (840)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3238)
Portable Handheld Ultrasound Scanner/Machine Digital +Convex Probe For Human

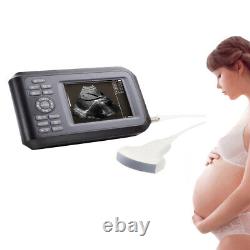

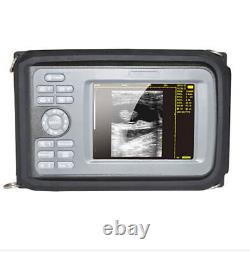

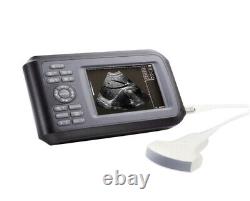

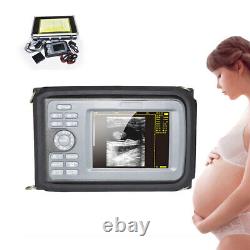
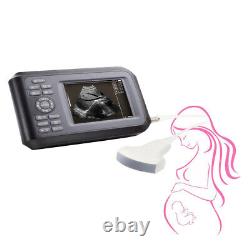
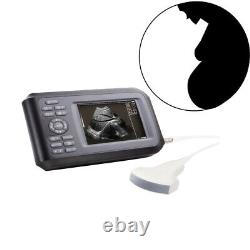


Display Modes: B, B+B, B+M, M, 4B. Image Conversion: distance, circumference, area, volume, EF rate, heart. Measurements: BPD, GS, CRL, FL, HC, AC, EDD, GA, FWVIDEO (PLAD, NTSC), USB2.0. One(1)R40/3.5mhz Convex Probe. Comments: date&time, name, sex, age, hospital.
Applications:Professional sofeware package in Obstetrics, Gynecology, Urology and Small parts. Statement:The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989. The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353. The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973.
Massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892. You pay us what you see on the invoice, i. Please make sure it is correct. Or replace item for you free of charge.This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "xinchengauto1" and is located in this country: US. This item can be shipped to United States.
- Brand: Carejoy
- Image Color: Black & White
- Country/Region of Manufacture: China
- Frequency: 2.5MHZ-7.5MHZ
- Display modes: B,B+B,B+M,M,4B

