
- Home
- Band
- 700 Mhz (2)
- Air (6)
- Am, Fm (6)
- Band (3)
- Cb, Uhf, Vhf (2)
- Digital (2)
- Hf, Uhf, Vhf (2)
- Hf, Vhf, Uhf (18)
- Hf, Vhf, Uhf, Cb (10)
- True I / Q Scanner (3)
- Uhf (169)
- Uhf, Hf (3)
- Uhf, Vhf (22)
- Uhf, Vhf, Cb (2)
- Uhf, Vhf, Hf (197)
- Uhf, Vhf, Hf, Cb (35)
- Uniden (4)
- Vhf (18)
- Vhf, Uhf (6)
- Vhf, Uhf, Cb (9)
- ... (4313)
- Brand
- Isbn
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (25)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzww (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2858)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (71)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (841)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3239)
- Weight
Portable Handheld Ultrasound Scanner/Machine Digital +Linear For Human Sale FDA

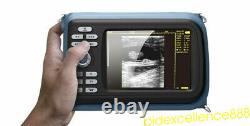
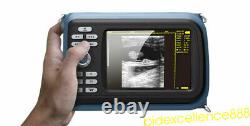






Display Modes: B, B+B, B+M, M, 4B. Image Conversion: distance, circumference, area, volume, EF rate, heart. Measurements: BPD, GS, CRL, FL, HC, AC, EDD, GA, FWVIDEO (PLAD, NTSC), USB2.0. One(1) L40/7.5Mhz HF linear Probe. Comments: date&time, name, sex, age, hospital.
Applications:Professional sofeware package in Obstetrics, Gynecology, Urology and Small parts. Probes: R40/5.0mhz Micro-convex Probe R10/6.5MHz Transvaginal Probe R40/3.5mhz Convex Probe.
Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is certified with the US FDA 510K No.
K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) NumberK132989. The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) NumberK180353.The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) NumberK141973. Massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) NumberK161892. The item "Portable Handheld Ultrasound Scanner/Machine Digital +Linear For Human Sale FDA" is in sale since Tuesday, August 18, 2020. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "bidexcellence888" and is located in Shenzhen.
This item can be shipped to United States.
- Model: HIGH RESOLUTION
- Country/Region of Manufacture: China
- UPC: 190891447364
- EAN: Does not apply
- Display: 5.5 inch TFT color LCD
- Certificate: CE
- Detectable depth: 70mm~220mm
- With: L40/7.5Mhz HF linear Probe
- MPN: Does not apply
- Brand: Unbranded

