
- Home
- Brand
- Features
- Battery Operated (4)
- Channel (3)
- Channel Monitor (42)
- Copier, Portable (8)
- Ctcss (19)
- Dcs (5)
- Digital (7)
- Digital Display (8)
- Digital Handheld (4)
- Gps (9)
- Handy Receiver (3)
- Motorola (4)
- P25 (3)
- Portable (10)
- Portable, Scanner (15)
- Printer (3)
- Radio (13)
- Scanner (17)
- Voice Activated (4)
- Wi-fi (6)
- ... (4631)
- Image Sensor
- Item Width
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (333)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2846)
- Product Line
USA 3.5MHz Micro-Convex probe For CONTEC Ultrasound Scanner Systems 2018 New



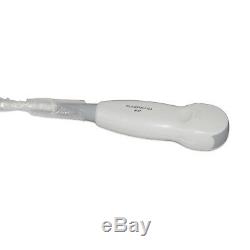


USA 3.5MHz Micro-Convex probe For CONTEC Ultrasound Scanner Systems 2018 New. 3.5 Mhz Micro-Convex Probe. The link is only for CMS600P2 Probe not include the Ultrasound Scanner. Please leave me your clear. All items will be dispatched within 2 bussiness day. We'd like to cooperate with you and declare the value of it. You want us to, so that it is more easier for you to clear the custom. The sale of this item may be subject to regulation by the U.
Food and Drug Administration and state and local regulatory agencies. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. The item "USA 3.5MHz Micro-Convex probe For CONTEC Ultrasound Scanner Systems 2018 New" is in sale since Wednesday, July 4, 2018.
This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical/Lab Equipment Attachments & Accessories\Medical Sensors & Ultrasound Probes". The seller is "medicalsystems2016" and is located in Elk Grove Village, IL.
This item can be shipped worldwide.- Model: 3.5MHz Micro-Convex Probe
- Modified Item: No
- Country/Region of Manufacture: United States
- Custom Bundle: No
- MPN: 69450401
- Brand: CON-TEC
- Non-Domestic Product: No
- Bundle Listing: Yes
- UPC: Does not apply

