
- Home
- Brand
- Connectivity
- Memory
- 100 Channels (192)
- 1000 Channels (240)
- 128 Channels (21)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (3)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4178)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (334)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2851)
- Series
- Ds3508 (12)
- Ds3508-er (5)
- Ds3578 (6)
- Ds3608 (10)
- Ds3608-sr (21)
- Ds4308 (7)
- Ds4308-hd (3)
- Li3608-er (5)
- Memor X3 (6)
- Motorola Ds3508 (3)
- Philips Dpm (5)
- Symbol Ds6707 (8)
- Symbol Ls2208 (3)
- U1-d (4)
- Voyager 1472g (5)
- Voyager Xp 1472g (3)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (15)
- Zebra Ds9208 (5)
- Zebra Li3608 (18)
- ... (4675)
- Type
- Barcode Scanner (173)
- Barcode Scanners (17)
- Barcode Scannner (12)
- Calling System (48)
- Digital Scanner (13)
- Earpiece (9)
- Hand Held (23)
- Handheld (201)
- Handheld Scanner (70)
- Mobile (10)
- Mobile Scanner (10)
- Pager System (11)
- Parts & Accessories (11)
- Portable (20)
- Portable / Handheld (839)
- Radio Scanner (10)
- Radio Scanners (9)
- Scanner (85)
- Ultrasound Scanner (10)
- Walkie-talkie (9)
- ... (3234)
Veterinary Color Ultrasound Scanner with 7.5 Mhz rectal Probes for Bovine, equine
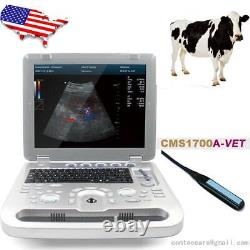

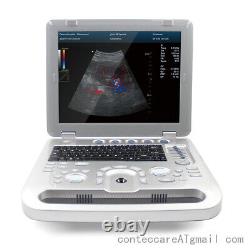





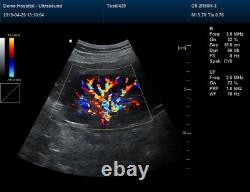
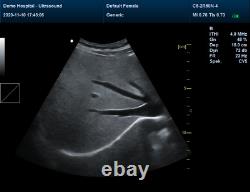


Veterinary Color Ultrasound Scanner with 7.5 Mhz rectal Probes for Bovine&equine. We are facotry, any product can give you 10% discount.
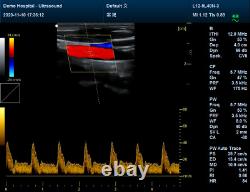
Please contact me Whatsapp:+8615243475232, conteccare AT gmail com. CMS1700A-VET is a color Doppler ultrasonic diagnostic device with high resolution, which has a powerful computer processing platform. It adopts Doppler ultrasound imaging technology, advanced image processing technology such as digital beam-forming technology, tissue harmonic imaging (THI), image speckle suppression, etc. And digital integrated graphic management system, and the internal professional measurement software package can fully meet the clinic diagnostic requirement.
Powerful function & Configuration Laptop, slim design, smart and light, easy to carry, convenient for examining. Built-in lithium battery 15 High resolution color LED monitor, high brightness, high contrast, wide visual, image clear and exquisite Menu operation, Interface language: Chinese/English can be switched.OB measurement: EDD and GA for Bovine, equine, ovine, canine, feline, goat, swine and llama. Automatically calculate backfat and lean percentage of swine. Light touch keyboard, trackball and encoder, easy operation for doctor. High speed USB port support high-capacity USB disk, and support the color laser printer print out all kinds of image and reports which make the output of diagnosis more convenient and simple.
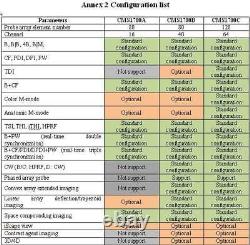
Rich clinical application function B+CF (Dual Images) B+CF/PDI/DPDI+PW (Triplex) Linear array deflection/Trapezoidal imaging (optional) Space compounding Imaging Wide scene imaging. (optional) Speckle noise removal technology Pulse inversion tissue harmonic imaging technology (iTHI).
1Display depth: = 300 mm 2Extended interface: VIDEO interface, S-VIDEO interface, RJ-45 interface, USB interface, VGA interface. Safety Type of protection against electric shock: class?
Equipment Degree of protection against electric shock: type B applied part Operating voltage: AC 100 V240 V Operating frequency: 50 Hz/60 Hz Power consumption: = 100 VA. Endo-rectal probe (7.5 MHz) One User Manual One power cord One adapter. Dimension: 370 mm (L) × 360 mm (W) × 80 mm (H) Weight: 6.5 kg. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. 100% satisfaction is our goal! Contec Medical Systems focusing on research, manufacture and distribution of medical instruments, was founded in 1992 as a high-tech company.We have us stock in IL, United States. We are looking forward to establishing a successful business relationship with you. Welcome to be our agent.
Only english user guide, if you need any other language, please contact me.
