
- Home
- Bluetooth
- Brand
- Memory
- 100 Channels (194)
- 1000 Channels (240)
- 128 Channels (21)
- 16 Gb (2)
- 200 Channels (6)
- 2500 Channels (77)
- 25000 Channels (25)
- 2gb Ram X 16gb Rom (5)
- 3000 Channels (3)
- 32gb (6)
- 400 Channels (16)
- 500 Channels (20)
- 6000 Channels (8)
- Lots (3)
- Micro Sd 4gb (2)
- Million (5)
- Unknown (6)
- 1800 (7)
- 5500 (4)
- 39000 (2)
- ... (4202)
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (335)
- Bearcat (25)
- Cms600p2 (512)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzww (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2878)
- Mounting
- Series
- Ds3508 (12)
- Ds3508-er (6)
- Ds3578 (6)
- Ds3608 (10)
- Ds3608-sr (21)
- Ds4308 (7)
- Li3608-er (5)
- Li3608-sr (4)
- Memor X3 (6)
- Philips Dpm (6)
- Symbol Ds6707 (8)
- Symbol Ls2208 (3)
- U1-d (4)
- Voyager 1472g (5)
- Xenon 1950gsr (3)
- Zebra Ds3608 (5)
- Zebra Ds3608-sr (15)
- Zebra Ds9208 (5)
- Zebra Li3608 (18)
- Zebra Tc56 (4)
- ... (4701)
Veterinary Handheld Digital Ultrasonic Scanner Convex Probe Animal Diagnosis FDA
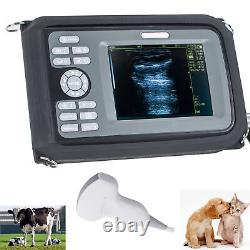
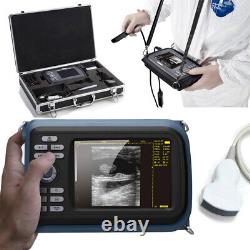


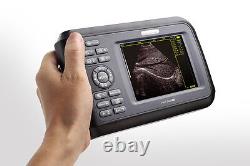
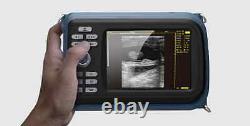
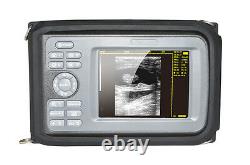
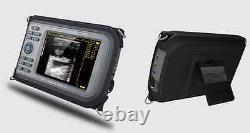






Cold Light Source & Cable. Syringe Pump and Infusion Pump. Veterinary ultrasound scanner + convex probe. Features for Digital Palm Smart Ultrasonic Scanner. HandScan V8 is a small, compact and battery powered Ultrasound Scanner for Veterinary applicationgs anyshere in the field Easy-to-use ultrasound scanner for animal diagnosis Ergonomically designed to sit comfortably Splash-resistant and dust tight Battery powered Light weight.
Screen Size: 5.5 inch, TFT color LCD Display Mode: B, B+B, B+M, M, 4B Gray Shade: 256 levels Scan Depth: 70240mm, adjustable Cine Loop: = 400 frames Built-in Memory: 64 images Focus Position: 2 points adjustable Annotation: date, clock, name, Sex, ID, Age full screen editor. Battery Working Time: = 3 hrsBattery Charging Time: = 2hr Net Weight: 700g Package: metallic case in a carton box Image Reservation: up/down, left/right Image Procession: Coded color, GAMA, Histogram And Image Smoothen, TCG: N. G (near gain) adjustable F.
G (overall gain) adjustable Language: English/Chinese switchable Measurement:: Distance, Circumference, Area, Volume and Heart Rate OB Measurement: Swine -- HL Sheep/Goat -- USD Cat - HD, BD Dog - BD, GSD, CRL, HD, BD AC adapter: 110240V, 50/60Hz. Two(2)shoulder and waist belts. ALL above will be packed in a carrying case. 6.5Rectal Linear Probe R50/3.5MHz Convex Probe R20/5.0MHz Micro-convex Probe L40/7.5MHz Linear Probe R60-80/4.0MHz Rectal Convex Probe. Biopsy Car Lighter Additional rechargeable battery.
Statement:The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is certified with the US FDA 510K No.
K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989. The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353. The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973.Massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892. You pay us what you see on the invoice, i.
Please make sure it is correct. Or replace item for you free of charge. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "fairtopshop888" and is located in this country: CN.
This item can be shipped to United States, Canada, Germany, Australia.
- Brand: Carejoy
- Model: V8
- Country/Region of Manufacture: China
- Weight: 700g
- Power: Both battery and AC Powered
- monitor: 5.5 inch TFT color LCD
- optional: Convex Linear Micro-Convex Probe
- standard Probe: rectal probe
- Dimensions: 230mm×120mm×42mm
- Applications: For Veterinary Vet Animal Dog sheep Goat pig Swine
- Scanning Depth: 70~240mm, adjustable
- Battery working: 3 hours
- carrying case: Yes
- belts: Two(2)shoulder and waist belts
- Image Storage: Built-in memory-64 images USB disk storage
- Optional 1: Biopsy / Car Lighter / Additional battery

