
- Home
- Brand
- Features
- Battery Operated (4)
- Channel (3)
- Channel Monitor (42)
- Copier, Portable (8)
- Ctcss (19)
- Dcs (5)
- Digital (7)
- Digital Display (8)
- Digital Handheld (4)
- Gps (9)
- Handy Receiver (3)
- Motorola (4)
- P25 (3)
- Portable (10)
- Portable, Scanner (15)
- Printer (3)
- Radio (13)
- Scanner (17)
- Voice Activated (4)
- Wi-fi (6)
- ... (4631)
- Image Sensor
- Item Width
- Model
- Aor Ar-dv10 (26)
- Bcd325p2 (67)
- Bcd396t (28)
- Bcd396xt (34)
- Bcd436hp (333)
- Bearcat (24)
- Cms600p2 (511)
- Cms600p2-vet (80)
- Cms600p2vet (91)
- Ds3608-sr00003vzcn (23)
- Handscan V8 (26)
- High Resolution (123)
- Pro-106 (39)
- Pro-651 (34)
- Pro-668 (50)
- Pro-96 (32)
- S50a (24)
- Sds100 (203)
- Trx-1 (134)
- Ws1040 (90)
- ... (2846)
- Product Line
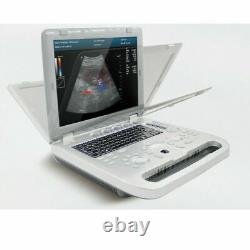
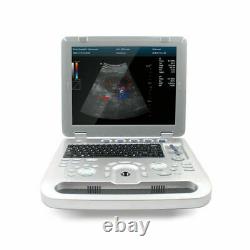
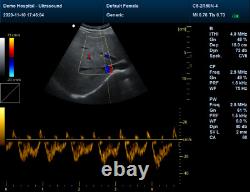
Veterinary Pet Bovine&equine color doppler Ultrasound Scanner vet 7.5mhz probe






Please pay within 2 days so as to avoid unnecessary delays and to ensure you get your item ASAP. An unpaid strike will be sent if the item has not been paid after 14 days. Take up about 1 month due to strict custom inspection. May take up to 4-8 weeks due to strict custom inspection. 100% satisfaction is our goal!
Client is reaponsible for the custom cleatance and custom duty. The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory.
If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. If you are not satisfied with the item when you receive it or haven't received item after 30 days, We will try our best to solve any problem.All emails will be answered within 24 hours on weekdays(but within 48hours on weekends). Only English user guide, if you need any other languange, please contact me.
This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Ultrasound Machines". The seller is "contecmed" and is located in this country: CN.
This item can be shipped worldwide.- MPN: 69450401
- Brand: CONTEC
- Ultrasound Machine Features: Color Doppler

